Chinese researchers this month report a 14% rate of caspofungin resistance in Candida glabrata, an organism that is also fluconazole resistant. In 67 intensive care units in China, 389 isolates of Candida from 244 patients were susceptibility testing using CLSI M27-A3 methodology.
50 isolates were C. glabrata and over 50% had fluconazole MICs of > 4mg/L (all either susceptible, dose dependent or resistant) and 14% had elevated caspofungin MICs (0.25mg/L).
These multidrug resistant isolates would be clinically responsive to amphotericin B (and possibly flucytosine) only.
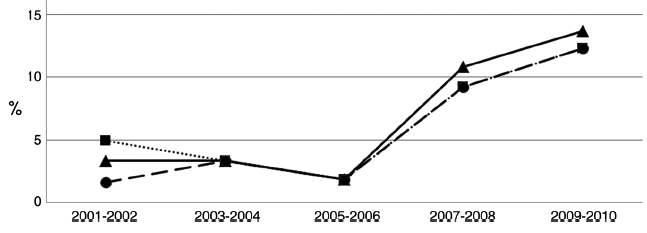
These data mirror the emergence of fluconazole and echinocandin resistant C. glabrata in the US, well illustrated by the experience at Duke University hospital. Over several years resistance to the echinocandins in C. glabrata climbed to 13% (figure below showing caspofungin resistance rates).
The ESCMID EUCAST breakpoints for C. glabrata effectively classifies all isolates as resistant arguing ‘C. glabrata is not a suitable target for fluconazole treatment’. It is not clear is dose escalation of fluconazole for SDD C. glabrata isolates is an effective therapeutic strategy, and most experts would use an alternative agent, with the possible exception of urinary infections. There have been recent adjustments in breakpoints for echinocandins in Candida as in vitro susceptibility methods are less precise in defining resistance than molecular methods that identify resistance ‘hot spot’ mutations. The Chinese study did not describe confirmation of resistance, as the US study did, by detecting mutations.
Nonetheless, the development of echinocandin resistance in Candida in so many institutions in China is worrisome. Candida glabrata grows more slowly in blood culture, often in the anaerobic bottle first, and the mortality is high. Infecting organisms that are resistant to both azole and echinocandin drugs leaves amphotericn B as the only choice available to treat these patients. However, the use of amphotericin will contribute to renal morbidity and death in some patients. A major concern is the culture negative patient and which agent is optimal in that setting.
Article, Liu et al , C. glabrata & Echinocandins, Alexander et al