 Echinocandin antifungal drugs are the ‘workhorses’ of antifungal therapy in hospitals. The WHO has endorsed their importance by adding them to the 2021 Essential Medicines List for low and middle countries, for both adults and children.
Echinocandin antifungal drugs are the ‘workhorses’ of antifungal therapy in hospitals. The WHO has endorsed their importance by adding them to the 2021 Essential Medicines List for low and middle countries, for both adults and children.
Initially launched in 2002 in Japan and USA, micafungin (Astellas) and caspofungin (Merck) were the first to market and then in 2006, anidulafungin (Pfizer) was also approved for use. The echinocandins kill almost all species of Candida and have good activity against Aspergillus, the commonest life-threatening fungi. The echinocandins are only effective if given intravenously.
The Global Action Fund for Fungal Infections (GAFFI) made the application to the WHO on behalf of many countries without access the life-saving antifungal medicines. Professor David Denning, chief Executive of GAFFI and of the University of Manchester, said: ‘This is good news for patients across the world. Although most countries have at least one echinocandin approved for use, prices are often high. Also, the multi-drug resistant hospital fungus Candida auris is difficult to treat without echinocandins.”
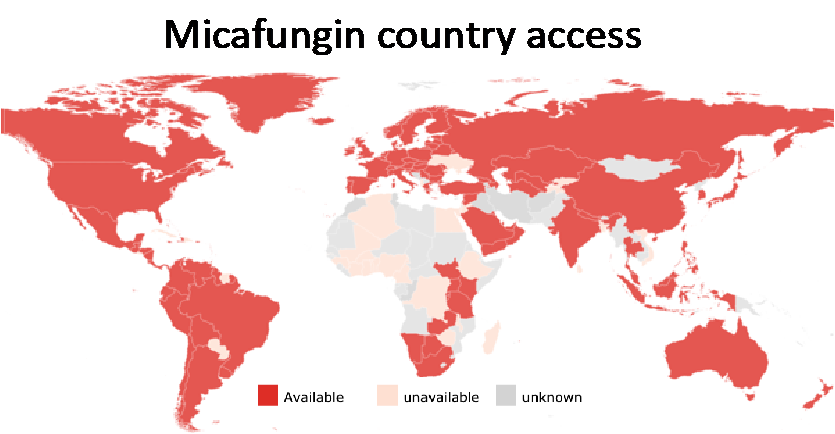
Micafungin is recommended ahead of caspofungin and anidulafungin, because of its current country availability and more published data for chronic pulmonary aspergillosis and premature infants.
 GAFFI recommends that the echinocandin class is considered essential therapy for:
GAFFI recommends that the echinocandin class is considered essential therapy for:
• Invasive candidiasis in adults and children
• Invasive candidiasis and candidaemia in neonates (micafungin only)
• Oesophageal candidiasis in patients unresponsive to azoles
• Invasive and chronic pulmonary aspergillosis in patients refractory to azole therapy, intolerant to azoles and in those with azole-resistant infections
• As prophylaxis in neutropenic patients in whom azoles are contra-indicated.
GAFFI’s application to the WHO.